Environmentally Friendly Heterogeneous Catalysis
Researchers at UCL are using a range of sophisticated computational tools available through local services, the Science and Engineering South Consortium and the UK’s National supercomputing service to simulate and predict the chemical processes that take place at the surfaces of metal and other material surfaces.
This study is concerned both with gaining insight into one of the most intriguing questions – the Origin of Life – and with finding a viable route to use a greenhouse gas as a raw material and yield useful organic feedstock molecules.
Although the bulk physical properties of many materials such as Platinum, Palladium and Iridium are well known, and can be used to gauge their appropriateness for specific applications; the surfaces of these materials exhibit qualities very different to the bulk. Surface qualities can be exploited to great effect in the efficient conversion of feed-stocks into valuable end products or the conversion of toxic substances into those that are environmentally benign which would simply not be possible if they were absent. The use of surface science in this manner is beneficial to society in mitigating pollution (e.g. car catalytic converters) and is an underpinning ingredient in the multi-billion pound UK chemical industry.
However, the transformation of feed-stocks using these surfaces (heterogeneous catalysis) is highly dependent on a number of conditions such as reaction temperature and pressure as well as the underlying surface and feed-stock being used. Traditionally the matching of these parameters to provide cost effective, energetically efficient and selective chemical products has been subject to experiment and significant trial and error by Researchers in academe and UK industry alike, this takes a long time to research for each reaction of interest and is exceptionally costly to undertake.
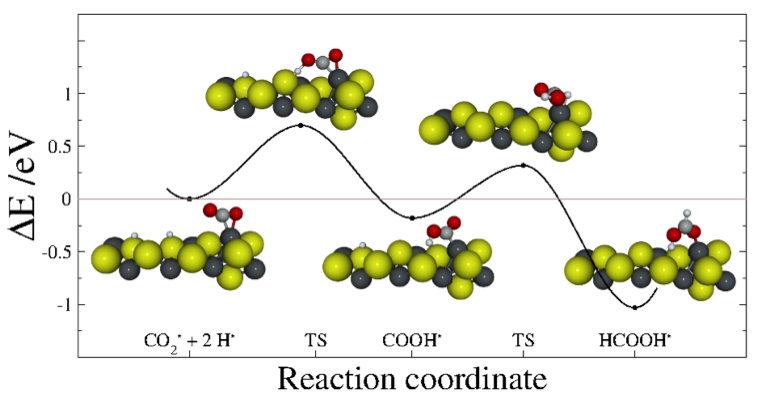
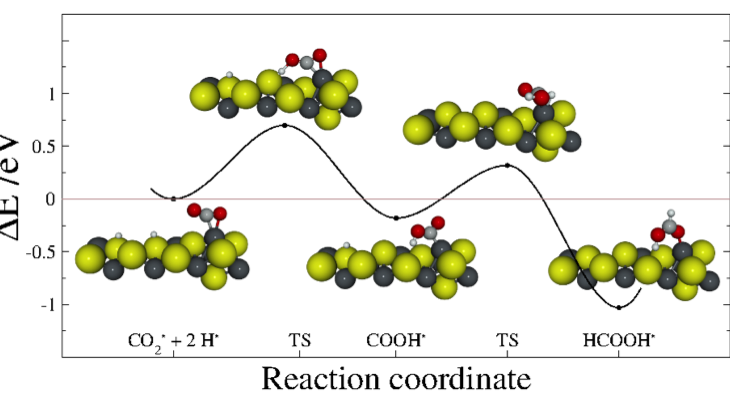
The initial objectives of our project were to optimise reactants and products to find transition states for a complete pathway transforming the greenhouse gas CO2 into an organic molecule. So far, we have the optimised structures of different intermediates coming from different reacting routes but because of the complexity of the system and intermediates leads to more routes worth to explore. Some of these intermediates lead to bigger molecules, containing more than one carbon, which means a new route for C−C synthesis. Although we need to complete the profile to provide a clear picture of the reaction by both thermodynamically and kinetically, we are now also able to describe the kinetic behaviour of the early reaction steps by mean the transition state energies. Both thermodynamic and kinetic data will provide information about the viability and velocity of the process for a large range of industrial conditions.
This is a computationally intensive research project and Prof. Nora de Leeuw and her team are using High Performance Computing to unravel the carbon dioxide transformation mechanism on a simulated “bio-inspired” catalyst. The importance of this reaction cannot be understated given the potential to convert and make useful this abundant and freely available greenhouse gas whilst reducing its impact on global warming. The insight gained into the actual mechanism of conversion will yield information which cannot be gained from experiment alone and provides vital clues to improve the catalyst’s efficiency.
IRIDIS is central in helping to unravel the different routes in a very efficient way and together with other supercomputer resources, provides the computational power this project demands. Even so, there remains the insatiable desire for higher levels of performance that not only reduces the computational time and therefore, enables collaboration with experimental investigations in parallel time-scale, but also increases reliability of the results. These are dependent on the level of theory used and the threshold of error accepted which is, in turn, directly influenced by
the package compilation, the parallelisation ability and the connection among the different computers.
As a bidirectional support, experimental and computational scientists use their tools to improve the knowledge and the welfare society; hence, scientific projects commonly combine those worlds. By utilising supercomputing capability, computational scientists may work closer to the same time-scales as experimentalist partners, decreasing the time lag and reliance on intuition and experience as lonely tools in the lab. Parallel collaboration minimizes the try-and-error old-school-science, wasting resources in reactant and solvents which usually pollutes the environment, by virtual simulations, highlighting the knowledge at nanoscale and real interaction between molecules observed in a screen desktop.
Future project stages to investigate industrial applications will necessitate the study of more complex and larger systems. Although the scale-up of the systems can still be based on results and methodologies used to date, an accurate description of these complex systems will ultimately be limited by the performance and availability of supercomputers to handle the complexity of simulations and enable throughput of results to enable progress to be made.
Continued local investment, shared regional systems and the UK National Service will provide the resources needed to continue this research and maintain the de Leeuw group’s competitiveness in this research area.
Published in the Journal of Chemical Physics, “A comparative DFT study of the mechanical and electronic properties of greigite Fe and magnetite”